Learning Outcomes
By the end of this lesson, students will be able to:
i. Define the term "solution" in chemistry, understanding the concept of a homogeneous mixture of two or more substances.
ii. Explain the concept of an aqueous solution, recognizing that water is the solvent in such solutions.
iii. Differentiate between solute and solvent, recognizing solute as the dissolved substance and solvent as the dissolving medium.
iv. Provide examples of solutions, solutes, and solvents found in everyday life.
v. Appreciate the significance of solutions in various scientific and technological fields.
Introduction
The world around us is filled with mixtures, and solutions stand out as a fascinating class of mixtures where different substances blend seamlessly, forming a homogeneous phase. Understanding the principles of solutions is essential to comprehending various chemical processes, from the preparation of beverages to the intricate workings of living cells.
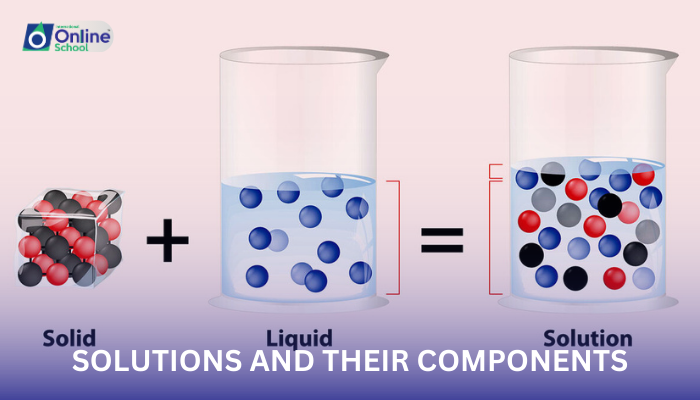
i. Solutions: A Homogeneous Blend
A solution is a homogeneous mixture of two or more substances, where the components are uniformly distributed throughout the mixture at the molecular or ionic level. This means that the individual components cannot be distinguished by physical means, such as filtration or sedimentation.
ii. Aqueous Solutions: When Water Takes Center Stage
An aqueous solution is a solution in which water acts as the solvent, the dissolving medium. Water, with its strong polarity and ability to form hydrogen bonds, is an excellent solvent for a wide range of substances.
iii. Solute and Solvent: Partners in a Homogeneous Mixture
In a solution, the solute is the dissolved substance, the component that is present in a smaller amount and is dispersed throughout the solvent. The solvent, on the other hand, is the dissolving medium, the component that is present in a larger amount and serves as the host for the solute particles.
iv. Everyday Encounters with Solutions
Solutions are ubiquitous in our daily lives:
Saltwater: A solution of salt (NaCl) dissolved in water, commonly known as saltwater, is an example of an aqueous solution.
Sugar Syrup: A solution of sugar dissolved in water, often used in baking and beverages, is another example of an aqueous solution.
Air: A mixture of gases, primarily nitrogen and oxygen, is an example of a gaseous solution.
Alloys: Mixtures of metals, such as brass (copper and zinc) or steel (iron and carbon), are examples of solid solutions.
Blood: A complex mixture of cells, proteins, and other substances, suspended in a liquid medium called plasma, is an example of a biological solution.
v. Significance of Solutions
Solutions play a crucial role in various scientific and technological fields:
Chemistry: Solutions are essential for laboratory work, chemical reactions, and analytical techniques.
Biology: The transport of nutrients, waste removal, and enzyme-mediated reactions within living cells occur in solution.
Pharmaceuticals: Drugs are often administered in the form of solutions to ensure uniform distribution and effective delivery to the target site.
Environmental Science: Understanding the behavior of pollutants and their interactions with solvents is crucial for environmental remediation efforts.
Industrial Processes: Solutions are widely used in various industrial processes, such as manufacturing, purification, and separation techniques.
Solutions, a fundamental class of mixtures, provide valuable insights into the behavior of substances and their interactions at the molecular level. By understanding the concepts of solute, solvent, and aqueous solutions, we gain a deeper appreciation for the homogeneity and versatility of these mixtures and their significance in various fields of science and technology.